Avian influenza is an infectious disease of birds caused by type A influenza viruses. Viruses are classified based on two surface proteins, Hemagglutinin (H) and Neuraminidase (N), which combine to form different subtypes (e.g., H5N1, H5N2, H7N3). Different subtypes, and strains within a subtype, vary in their ability to cause disease in birds. Avian influenza viruses are categorized as highly pathogenic (HP) or low pathogenic (LP) based on their ability to cause disease in domestic poultry. Historically, viruses of H5 and H7 subtypes have been more likely to become highly pathogenic. When viruses of different subtypes occur in the same host, virus reassortment may occur resulting in a new subtype. Viruses also may change from low pathogenic to highly pathogenic; this change typically occurs in the domestic animal host.
Avian influenza viruses naturally circulate in waterbirds including waterfowl and shorebirds, with or without clinical signs. Avian predators or scavengers, including eagles, other raptors, corvids, gulls, or vultures, may be exposed when feeding on infected waterbirds especially during mortality events (e.g., avian cholera, avian botulism). In poultry, HPAI may cause significant mortality. Birds raised in captivity, such as other gallinaceous birds (turkeys, pheasants, grouse, quail) and waterfowl (ducks, geese, swans), may also be at high risk of acquiring and transmitting the virus. Avian influenza viruses are shed in bodily fluids such as saliva, nasal secretions, and feces. They can be transmitted directly from an infected bird or indirectly through people or objects contaminated with virus particles (e.g., animal crates, feathers, food/water, clothing, etc.). Signs in sick birds may include neurological signs (head tilt, tremors, weakness, incoordination), respiratory distress, inappetence, and diarrhea.
Avian influenza viruses gained increased public attention in the early 2000s when HPAI H5N1 outbreaks caused significant losses of domestic poultry in Southeast Asia. This virus has continued to cause periodic outbreaks in domestic poultry and occasionally wild birds in Asia, Europe, and Africa.
In late 2014 and 2015, different strains of avian influenza viruses, including both highly pathogenic and low pathogenic viruses, were identified in both wild and domestic birds in North America including in California. Highly pathogenic H5N2 and H5N8 viruses caused outbreaks in domestic poultry in Canada and the United States, with occasional detections in wild birds in areas with outbreaks in domestic poultry. The H5N8 viruses detected in North America were the result of genetic reassortment of viruses of Asian lineage with those of American lineage. Low pathogenic H5N2 and H7N3 viruses also were periodically detected in domestic poultry operations in the United States and elsewhere.
In December 2021, highly pathogenic avian influenza (HPAI) H5N1 of Eurasian-lineage was detected in domestic poultry and wild birds along the Atlantic coast of Canada. In January and February 2022, detections were made for the first time in wild birds and domestic poultry in the eastern United States. Following its initial detection, the virus has spread to nearly every state with numerous detections in both domestic poultry and wild birds. Prior to its detection along the Atlantic coast, Eurasian HPAI H5N1 activity had been on the rise across Europe in domestic poultry and wild birds since October 2021. In the U.S., detections have been made during surveillance in apparently healthy hunter-harvested and live-sampled waterfowl, as well as in sick and dead waterfowl and other wild birds found individually or during mortality events. Although avian influenza viruses naturally circulate among waterbirds, the strain of H5N1 currently in circulation in the U.S. and Canada has been causing illness and death in a higher diversity of wild bird species than during previous avian influenza outbreaks. The virus also remains highly contagious for domestic poultry. Avian predators and scavengers may be exposed to avian influenza viruses when feeding on infected waterbirds. Infection with avian influenza viruses among songbirds, including many common backyard birds, appears to be rare.
Related Documents

Low pathogenic avian influenza viruses naturally circulate in waterbirds such as snow geese and northern pintail ducks.

A species-adapted strain of avian paramyxovirus-1 periodically causes mortality events in double-crested cormorants.

A pigeon-adapted strain of avian paramyxovirus-1 has recently emerged in non-native Eurasian collared doves in California posing a potential disease risk to native species.
Avian Orthoavulavirus serotype 1, formally called Avian Paramyxovirus serotype 1 (APMV-1), are a group of viruses that encompass a large number of strain variants that may affect a diversity of both domestic and wild birds. The virulence of strains are classified as mild (lentogentic), moderate (mesogenic), or very virulent (velogenic). When virulent APMV-1 infections occur in domestic chickens, the disease is often referred to as virulent Newcastle Disease. This strain variant is highly contagious among chickens and may cause significant mortality. Among free-ranging birds, the two most common variants include a species-adapted APMV-1 in native double-crested cormorants and Pigeon Paramyxovirus-1 (PPMV-1), a strain variant adapted to columbids primarily causing disease in non-native rock pigeons and Eurasian collared doves.
Avian Avulaviruses-1 are shed in body fluids such as saliva, nasal secretions, and feces and can be transmitted directly from a sick bird or indirectly through people or surfaces contaminated with these materials (e.g. food, water, soil, perches, feathers, clothing, footwear, vehicles). Clinical signs can vary depending upon the species affected and the strain type. In general, signs typically develop within 2-15 days after exposure and may include tremors, drooping wings, partial paralysis, twisting of the head and neck, or circling. Birds often act lethargic and may have ruffled feathers or difficulty breathing.
Strains of APMV-1, including the pigeon variant PPMV-1, are relatively hardy and can survive in water, soil, or on structures like bird feeders and perches for days to weeks under appropriate conditions. Transmission most commonly occurs at locations were birds are in close contact including sources of food and water as well as roosting or breeding areas. During outbreaks of PPMV-1 in non-native Eurasian collared doves, water sources including birdbaths, fountains, and decorative ponds, are common sources of transmission. Disease outbreaks in non-native Eurasian collared doves may put our native band-tailed pigeon and doves at risk of infection when using these locations.

Avian pox lesions around the eye and bill of a California towhee.

Avian pox lesions on the feet of a red-tailed hawk.
Avian pox is a virus that causes wart-like, raised growths or lesions, on the unfeathered skin of both domestic and wild birds. These growths often develop on the skin around the eyes or the bill, or on the legs and feet. Avian pox is often not directly lethal; however, it can contribute to serious debilitation. The growths may affect a birds’ ability to find food leading to starvation or the growths can become abraded leading to secondary infection with bacteria or fungi.
Some strain variants of avian pox are likely specific to certain avian species while some may be transmitted between different avian species. Avian pox virus is transmitted through direct contact from an infected bird, or from contaminated surfaces like bird feeders or perches. Biting insects like mosquitos also can transmit avian pox. For this reason, cases of avian pox tend to occur in seasons and areas with higher mosquito activity.
In California, reports of avian pox infections tend to increase during the summer and fall and often involve juvenile birds, who are most susceptible to infection. Species most commonly reported include corvids like crows and ravens, doves, and a variety of songbirds. Raptors, such as red-tailed hawks, may also be impacted by avian pox and develop lesions on their feet and legs after handing infected avian prey or contact with an infected surface.
West Nile virus (WNV) is one of many arboviruses that are transmitted by an arthropod vector. West Nile virus was first detected in the northeastern United States in 1999 and in California in 2002. West Nile virus, with one of the broadest host and vector ranges, has been isolated in numerous species of birds, reptiles, amphibians, and mammals including humans.

Raptors, such as this Cooper's hawk, can aquire West Nile virus infection through their prey or a mosquito bite.
Generally, WNV is transmitted to the host through the bite of an infected mosquito mainly within the genus Culex. The virus replicates in the hosts blood and may be transmitted to a new mosquito when it bites the infected host. The virus is maintained primarily through transmission between birds and mosquitos; however, not all bird species produce viremias, or copies of the virus in the bloodstream, capable of infecting a mosquito. Mammals such as humans and horses are considered dead-end or accidental hosts, meaning they do not produce viremias capable of infecting mosquitos; however, they may develop the disease and exhibit clinical signs of infection.
Infection can range from mild to severe depending upon the host species and other complicating factors such as age. The disease is typically characterized by encephalitis, the swelling of the brain, or meningitis, the swelling of the lining of the nervous system. Infection may result in neurological dysfunction including uncoordinated movements, abnormal positioning of the head and neck, and/or paralysis of the limbs. Corvids including magpies, jays, crows, and ravens are highly susceptible to the disease as well as some species of raptors such as Cooper’s hawks, which may acquire the infection through mosquito bites or through the ingestion of infected prey.
West Nile virus infections are linked with mosquito activity, which is highest during the summer and fall. Eliminating or draining sources of standing water such as birdbaths, rain gutters, buckets, or tires can help reduce mosquito breeding activity. During peak seasons, public health agencies may conduct WNV testing on dead birds to monitor for virus activity in different geographic areas.
Avian cholera is an infectious disease caused by the bacterium Pasteurella multocida. Avian, or fowl cholera, had been recognized as a disease of domestic birds in North America since the 1800s. Outbreaks in wild waterfowl were first reported in the United States in the 1940s. Outbreaks are usually reported annually during the winter along the major migratory flyways including the Pacific Flyway through California. Annual to near-annual outbreaks occur in some locations while other locations may have an outbreak only once or every few years.

Avian cholera causes annual winter mortality events in waterfowl which may involve hundreds of birds in a single location.
Outbreaks in California are often reported during the winter months when large numbers of migratory waterfowl congregate and temperatures turn cold (freezing or near freezing). The combination of large numbers of susceptible birds and the physiological stress caused by cold temperatures and increased social interactions are thought to prompt an outbreak. Additionally, some individual birds may carry the bacteria with them through migration and shed the bacteria in feces and other body fluids.
Outbreaks most commonly involve waterfowl like ducks and geese although other waterbirds like shorebirds, waders, and gulls also are susceptible to infection. When large numbers of susceptible birds are present, transmission can be rapid resulting in the deaths of hundreds to thousands of birds in a single event. The presence of infected carcasses on the landscape can contribute further to disease spread among waterfowl and other waterbirds while avian predators and scavengers such as raptors, crows, and gulls may become infected when feeding on sick birds or carcasses.
Infected individuals typically deteriorate rapidly. Signs of infection may include matting of feathers with fecal material, watery yellow feces, bloody discharge from the nose or vent, convulsions, incoordination, and lethargy.
Avian mycoplasmosis is a disease caused by the bacterium Mycoplasma gallisepticum. While some strains of M. gallisepticum cause disease in domestic poultry, different strains have been found to cause disease in wild birds, most notably within the songbird family Fringillidae, which includes the finches. Infected songbirds typically develop conjunctivitis characterized by red, swollen eyes and may die from the disease or complications such as starvation. Recent research has identified other species of Mycoplasma, including M. sturni, which also may cause conjunctivitis in different avian species.

Mycoplasmosis, characterized by red swollen eyes as in this house finch, can spread rapidly among birds at birdfeeders.
Outbreaks of avian mycoplasmosis in house finches were first observed in 1994 in their introduced range in the eastern United States. Since that time, the disease has spread westward to house finches in their native range in the western United States. In recent years, near-annual outbreaks have occurred in California and elsewhere. These outbreaks often involve large numbers of house finches, American goldfinches, and lesser goldfinches infected with M gallisepticum. Conjunctivitis also has been observed periodically in corvids, mockingbirds, and other songbirds; although it is less clear with what Mycoplasma species they are infected.
Transmission of Mycoplasma occurs through direct contact with infected birds, or indirect contact with surfaces contaminated with body secretions. In California songbirds, avian mycoplasmosis is almost exclusively reported at bird feeders where sick birds, often with squinty eyes and ruffled feathers, may sit for hours to days before succumbing to death. Tube bird feeders are especially problematic because eye discharge can easily contaminate the feeder surface as the bird inserts its head into the hole to reach the seed. Multiple individuals of different species may be at risk of acquiring infection during a single days feeding. During the spring breeding season, infected parents may also transmit the infection to their offspring resulting in the complete failure of that reproductive effort.

Salmonellosis causes periodic mortality events in pine siskins and is spread primarily at bird feeders.
Salmonellosis is an infection caused by species of bacteria within the genus Salmonella. The genus, which contains over 1,000 species and 2,500 distinguishable serotypes, has a worldwide distribution and may infect numerous animals including birds, reptiles, and mammals. In wild birds, Salmonella infections may result in subclinical infection with no apparent impact to an individuals’ health to chronic infection with some debilitation to acute enteritis, septicemia and death. In California, and elsewhere in the United States and Canada, outbreaks are observed periodically in migratory songbirds and are often the result of infection with serotype Salmonella typhimurium.
Salmonella bacteria infect the intestinal tract and are primarily transmitted by the fecal-to-oral route. The probability of infection is thus increased in locations with significant buildup of fecal material such as at bird feeders and birdbaths. Infections are reported frequently in finches, and most notably pine siskins and goldfinches, using bird feeders during the winter. However, other birds such as sparrows that are in close contact with infected finches at artificial feeding sites also are at risk of infection. Signs of infection include ruffled feathers or a “puffed-up” appearance, weakness, reluctance to move, labored breathing, and diarrhea. Salmonellosis outbreaks in pine siskins and goldfinches often results in the death of several of thousands of birds during a single winter.
Aspergillosis is a respiratory disease caused by the inhalation of fungal spores of the genus Aspergillus, most commonly A. fumigatus. These fungal spores are widespread in the environment and associated with soil, decaying vegetation, moldy birdseed, and agricultural waste grains. Aspergillosis may occur as a chronic infection in individual birds or as an acute infection affecting one to many birds in a single location.

Nestlings are at risk of developing fatal Aspergillosis if the nest box is not properly cleaned at the end of the season.
Aspergillus species grow best under moist conditions and produce spores when temperatures reach the mid-to-upper 70s. The spores are then released into the environment where they are inhaled by birds, becoming dispersed throughout their respiratory system. Immune compromised individuals and juveniles are at a higher risk for developing the disease. The development of disease in healthy individuals is rare except when exposed to large numbers of spores. Additionally, spores can reside in the respiratory system for extended periods and become active once the bird becomes immune compromised through other disease or injury.
Birds with chronic Aspergillosis may develop exercise intolerance, poor body condition, and have difficulty or labored breathing. Acute infections occur suddenly often without any signs of disease. Infections have been documented periodically in birds that utilize nest boxes such as bluebirds and barn owls. As such, it is important to thoroughly clean nest boxes at the end of the breeding season because the nest material could harbor Aspergillus spores, which could harm the next seasons’ young. Acute infections also have occurred in various species of songbirds congregating at bird feeders or birdbaths resulting in the deaths of several individuals in a localized area while isolated cases of Aspergillosis are often diagnosed in immune compromised or injured raptors and seabirds.

Waste grain may harbor fungal spores that could result in the infection of birds that eat it such as migratory waterfowl.

Avian trichomonosis causes near-annual mortaltiy events in our native band-tailed pigeons. Sick birds often appear weak and reluctant to fly.

Avian trichomonosis in our native band-tailed pigeons results in oral lesions that can block the passage of food or the airway.

Native mourning doves also are highly suceptible to avian trichomonosis.
Avian trichomonosis is a disease caused by a protozoan parasite typically Trichomonas gallinae. The parasite invades the mucous membranes of the birds’ upper digestive tract causing the tissue to die leading to the development of caseous lesions, meaning “curd-like” in appearance. As infection progresses, the lesions will eventually block the passage of food resulting in starvation or block the airway causing the bird to suffocate. Infected birds often appear lethargic, have ruffled feathers or a “puffed-up” appearance, labored or open mouth breathing, and/or repeated swallowing. As the disease progresses, birds become emaciated and are often unable to sustain flight.
Trichomonas parasites are primarily transmitted through contaminated water. When an infected bird drinks, numerous parasites may be released into the water. Because these parasites can survive in water for hours to days under appropriate conditions, many birds have the potential to become infected when they drink from the same water source. A shared water source that acts to concentrate the parasites such as birdbaths, fountains, and horse troughs are important locations for transmission during outbreaks. Transmission also may occur when an infected bird regurgitates seed that is then consumed by another bird or parents can transmit the parasite to their offspring during feeding. Raptors that prey on infected birds also may become infected and die.
Many species of birds are susceptible to infection with Trichomonas parasites; however, not all birds will develop the disease, but they may aid in parasite transmission. In California, native band-tailed pigeons and mourning doves are especially susceptible to the disease with near-annual outbreaks occurring in both species. Outbreaks in band-tailed pigeons typically occur during the winter when nearly the entire Pacific Coast population is overwintering in California, forming large flocks where the disease can spread rapidly. Outbreaks in mourning doves typically begin in the spring as immunologically naïve juveniles enter the population. Total mortality during avian trichomonosis outbreaks may number into the thousands in a given year for both band-tailed pigeons and mourning doves. Artificial sources of water are the most important site for disease transmission for these species.
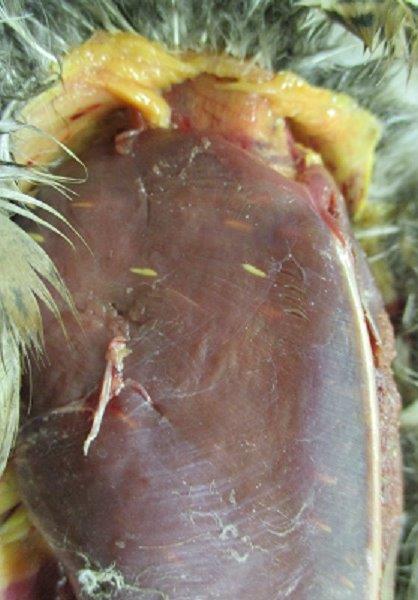
Some Sarcocystis infections result in the development of tan-colored cysts in the breast muscle of affected birds. In ducks, this condition is often referred to as rice breast.
Sarcocystosis is an infection caused by a protozoan parasite of the genus Sarcocystis. Sarcocystis parasites require two hosts to complete their life cycle, an intermediate host and a definitive host. Intermediate hosts are typically prey species (herbivore or omnivore) and may include species of songbirds, pigeons and doves, ducks, or small mammals such as rodents and squirrels. Definitive hosts are typically predator species (carnivore) and may include species of raptors and larger mammals such as skunks and raccoons. Many different avian and mammalian species have been documented as either intermediate or definitive hosts for different Sarcocystis parasites.
Intermediate hosts become infected through the ingestion of sporocysts, a life stage of the parasite, in contaminated water or food. Inside the intermediate host, the sporocysts undergo asexual reproduction and make their way into the muscle where they form sarcocysts, for which the organism is named. When the muscle is consumed by the appropriate definitive host, the parasite undergoes sexual reproduction producing oocysts, or eggs, that are passed in the feces and develop into sporocysts.
Sarcocystosis is an infection caused by a protozoan parasite of the genus Sarcocystis. Sarcocystis parasites require two hosts to complete their life cycle, an intermediate host and a definitive host. Intermediate hosts are typically prey species (herbivore or omnivore) and may include species of songbirds, pigeons and doves, ducks, or small mammals such as rodents and squirrels. Definitive hosts are typically predator species (carnivore) and may include species of raptors and mammals such as opossums and skunks. Many different avian and mammalian species have been identified as either intermediate or definitive hosts for different Sarcocystis parasites. The severity of clinical disease in intermediate hosts will vary depending upon the species of Sarcocystis and the number of parasites ingested. Avian definitive hosts infected with Sarcocystis parasites do not typically appear sick.
Intermediate hosts become infected through the ingestion of parasite eggs in contaminated water or food. Inside the intermediate host, the eggs undergo asexual reproduction and make their way into the muscle where they form sarcocysts, for which the organism is named. When the muscle is consumed by the appropriate definitive host, the parasite undergoes sexual reproduction producing oocysts, or eggs, that are passed in the feces.
A commonly observed form of Sarcocystosis occurs in waterfowl. Dabbling ducks are an intermediate host of S. rileyi and sarcocysts, which resemble grains of rice, may be visible in the pectoral muscles. Duck hunters commonly refer to this condition as “rice breast.” Apart from the sarcocysts in the muscle tissue, these intermediate hosts often appear otherwise healthy.

A pigeon-adapted strain of Sarcocystis has recently emerged in non-native rock pigeons causing neurological dysfunction.
In 2017, S. calchasi was identified in California as the cause of neurological disease in free-ranging rock pigeons during a mortality investigation. Infected rock pigeons had microscopic sarcocysts present in the muscle as well as encephalitis resulting in neurological dysfunction including twisting of the head and neck, incoordination, difficulty standing and flying, and death. Infection was identified in multiple rock pigeons in several different locations during this outbreak, which is unusual for Sarcocystis parasites. Given that rock pigeons are widespread, S. calchasi is also likely widespread in California. Sarcocystis calchasi also been detected elsewhere in the United States, and in Europe, primarily in free-ranging and captive rock pigeons. It is unknown if our native band-tailed pigeons and doves are susceptible to infection.
In 2019, S. calchasi was detected for the first time in a seabird, the Brandt’s cormorant, during a mortality event along the southern California coast. Infected Brandt’s cormorants developed encephalitis similar to the rock pigeons. Sarcocystis calchasi outbreaks have been detected in Brandt’s cormorants along the southern coast annually since its initial detection in 2019. While an individual bird may become infected with the parasite at any time of year, mortality events involving multiple birds, are most often reported during the late winter and spring.
Recently, Cooper’s hawks and red-tailed hawks were identified as definitive hosts for S. calchasi in California. The parasite likely cycles between rock pigeons (intermediate host) and the raptors (definitive hosts). The parasite eggs are shed in the feces of the raptor where they are ingested by the rock pigeon. Once infected, the parasite form sarcocysts in the muscle tissues of the rock pigeon, and then if eaten by the raptor, the life cycle begins again. The Brandt’s cormorants are likely a dead-end host since they are not regularly predated by raptors given their large size. Presumably the eggs shed in the raptor feces are washed into the ocean during winter rains where they are ingested directly or indirectly by the Brandt’s cormorants.
 Avian botulism often occurs in warm water with high organic content, such as a city park pond.
Avian botulism often occurs in warm water with high organic content, such as a city park pond.
Avian botulism is a naturally occurring neurotoxin produced by the bacterium Clostridium botulinum. There are seven different types of biotoxins, named types A through G. In wild birds, type C botulinum toxin is most frequently reported and primarily affects ducks and sometimes shorebirds and gulls. Type E botulinum toxin is less frequently reported and affects fish-eating birds like pelicans and loons. Clostridium botulinum forms spores that can remain in the environment for years. The toxin is produced when the spores begin to germinate and grow under appropriate conditions, which typically occur during the summer and early fall.
Conditions necessary for bacterial growth that produces the type C toxin includes warm to hot temperatures and the presence of decomposing organic matter such as aquatic vegetation, bird feces, invertebrates, or fish reducing oxygen levels. Birds inadvertently ingest the toxin as they feed. The cycle is perpetuated when birds begin to die and decompose providing more substrate for bacterial growth. In California, avian botulism outbreaks are most often reported at city or county park ponds and water treatment plants. These locations often have high numbers of susceptible ducks and abundant organic matter. For type E avian botulism, the bacteria are present in the gastrointestinal tract of various fish, the toxin is produced as the bacteria grow, and fish-eating birds ingest the toxin when feeding on affected fish.
Once ingested, the toxin affects the birds’ peripheral nervous system causing progressive paralysis. Paralysis is apparent first in the legs and wings, followed by the inability to hold the head up (often referred to as limberneck), closed eyelids, decreased respiratory and cardiac function, resulting in death. The amount of toxin consumed directly relates to the severity of the paralysis and degree of mortality observed at a location. In some cases, sick birds may recover if removed from the source of the toxin and provided with fresh water and a safe environment in which to recover.
Related Resources

Warm temperatures and high nutrient content in reservoirs and streams may contribute to more frequent algal blooms with the potential to produce harmful toxins.

Waterbirds and other animals may encounter and be affected by harmful algal blooms.

Domoic acid can accumulate in small feeder fish that are the prey of marine mammals and seabirds like California sea lions and Brandt's cormorants.
Algal toxins encompass a wide variety of naturally occurring toxins produced by different species of algae and cyanobacteria. During periods of algal growth or blooms, toxins may be released that can be hazardous to animals and people. These blooms, often referred to as harmful algal blooms or HABs, occur worldwide. The algal toxins that are most frequently reported in California wildlife include cyanotoxins produced by cyanobacteria and domoic acid produced by marine diatoms.
Cyanobacteria are a group of photosynthetic bacteria. While they are not technically algae, they are commonly referred to as blue-green algae. Named for the color of their blooms, some varieties may actually produce green, blue, reddish-purple, or brown blooms. Cyanobacteria grow best in lakes, ponds, reservoirs, estuaries, and slow-moving creeks or rivers. Under appropriate conditions, which often include warm water temperatures and increased nutrient loading of phosphorus or nitrogen from human activities, the cyanobacteria may bloom. During the bloom, growth accelerates often forming green-colored mats or layers of scum on the water’s surface. Because water temperature influences growth, blooms typically occur during the summer and fall. As the cyanobacteria cells die or break open during wind or other water activity, the toxin may be released into the water. Wild birds and other animals can then be exposed to the toxin when they have contact with or ingest the water. Waterbirds such as ducks, gulls, grebes, and waders, may be at a higher risk for exposure to these toxins. Interestingly, toxins are not always produced during a bloom and toxins may be present in the absence of an obvious bloom. Two types of toxins produced by cyanobacteria include microcystins, a hepatotoxin, and anatoxin, a neurotoxin.
Domoic acid is a neurotoxin produced by marine diatoms or microalgae. These diatoms naturally occur in the marine environment. Under appropriate conditions, the diatoms produce the toxin. Small fish like anchovies and sardines ingest the toxin when feeding on the diatoms. The toxin subsequently affects fish-eating seabirds such as brown pelicans, cormorants, and loons as well as marine mammals when they feed on the fish, which acts to concentrate the toxin. Affected birds may exhibit muscle tremors, side-to-side head movements, uncoordinated flying or walking, toe clenching, twisting of the head and neck, vomiting, and/or inability to right themselves.
Diagnosing algal neurotoxins in a bird can be challenging because available tests require the detection of the toxin in stomach or gastrointestinal contents. Birds affected by the toxin may regurgitate food or not eat for days before they die, as such, sufficient toxin may not be present in their stomach to produce a positive test. Conversely, mycrocystins are typically associated with liver damage; however, if an animal dies quickly after exposure, characteristic lesions may not be evident. In some cases, a suspected diagnosis is made based on clinical signs exhibited by the birds before death and environmental conditions, which may include the presence of algal mats and/or the detection of the algal species in the water where mortality has occurred.
Visit the California State Water Resources Control Board for information on the HABs  monitoring program or to monitoring program or to  report a suspected bloom. The California Department of Public Health provides information on the report a suspected bloom. The California Department of Public Health provides information on the  marine biotoxin monitoring program. marine biotoxin monitoring program.

Following a hard freeze, the sugars in berries remaining on trees during winter can ferment resulting in the production of alcohol. Birds, such as American robins that feed on fermented berries, may then become intoxicated.

Fruit eating birds, such as cedar waxwings, are at risk of acute alcohol toxicity or trauma during impaired flight, when they feed on fermented berries.
Ethanol poisoning is a natural toxin produced within berries through fermentation. Ethanol poisoning typically occurs in California after the first hard freeze in late December or early January. The sugars in berries that have remained on plants from the summer begin to ferment producing alcohol as a byproduct. Birds are not aware this change has occurred and continue to feed on the berries as they normally would. Birds that consume these berries essentially become drunk or intoxicated. Affected birds may exhibit uncoordinated movements and have difficulty flying.
While death due to acute poisoning is possible, the majority of deaths associated with these events are due to traumatic injuries resulting from collisions with windows or other structures. Fruit eating species are susceptible and often include cedar waxwings and thrushes such as American robins, hermit thrushes, and varied thrushes. Mortality may occur in close proximity to the tainted berries or at a seemingly random location and is dependent upon the amount of ethanol ingested and how quickly it is metabolized.
When a gregarious species (e.g. cedar waxwing) is affected, an entire flock of a single species may succumb to poisoning at more or less the same time and location. When a more solitary species (e.g. varied thrush) is affected, individual deaths may occur over a wider geographic area during a given winter. Unfortunately, there is no test to confirm ethanol poisoning in birds so diagnosis is made based on clinical signs exhibited by the birds before death, environmental conditions, and/or the presence of berries in the birds’ digestive tract.

Anticoagulant rodenticide exposure is a widespread concern for predators and scavengers like this great horned owl.

Anticoagulant rodenticides acquired through feeding on contaminated prey can cause fatal bleeding in predators such as in the leg of this great horned owl.
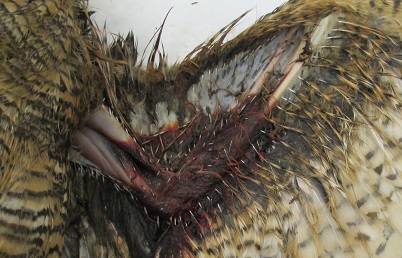
Anticoagulant rodenticides may cause a minor injury to become a lethal injury through fatal bloodloss such as in the wing of this great horned owl, or impair the predators ability to hunt for prey leading to starvation.
Anticoagulant rodenticides are pesticides that were developed to control rodent populations by predisposing them to fatal hemorrhage by disrupting blood-clotting factors. As rodents developed resistance to first generation anticoagulant rodenticides like warfarin, second generation anticoagulant rodenticides (SGARs) were introduced including brodifacoum and bromadiolone, which are more toxic and persist longer in tissues. A rodent may continue to feed on bait for a few days after initial exposure. If a predator or scavenger subsequently consumes the rodent, they will be secondarily exposed to the SGARs. Monitoring in California has demonstrated that roughly 70 to 90% of raptors tested had exposure to SGARs. Some wildlife species such as kangaroo rats, woodrats, squirrels, skunks, and raccoons, may ingest the bait directly resulting in poisoning.
Second generation anticoagulant rodenticides are commonly used in urban and suburban settings where human structures and activities provide shelter and food for rodent pests. Activities like bird feeding or raising backyard chickens may inadvertently attract rodents leading to the use of rodenticides potentially harming local wildlife and domestic pets if they consume a rodent that has ingested bait. These toxins are stored in the animals’ liver where repeated exposure can result in a lethal dose. Sublethal levels also may impact wildlife by turning minor injuries into lethal wounds.
Long-term rodent control is best accomplished though habitat modifications such as sealing holes in buildings and removing attractants such as pet food, fallen fruit, wild bird and chicken seed, and overgrown vegetation or debris. For more information, please visit the Departments’ rodenticide web page.

Scavengers, like these turkey vultures, are at risk when euthanized animals are disposed of improperly through incineration or deep burial.
Pentobarbital is a veterinary drug used to humanely euthanize animals including pets such as dogs and cats, horses, goats, cattle, poultry, and other livestock. During euthanasia, the drug is quickly distributed throughout the animals’ body including into the organs and muscle tissues. Secondary poisoning of wildlife occurs when the animal remains are disposed of improperly and scavengers feed on the animal exposing them to the euthanasia drug.
Pentobarbital poisoning of wildlife is one-hundred percent preventable. The law requires that animals, chemically euthanized with pentobarbital, be cremated or buried at least 3 to 4 feet deep to prevent exposing scavenging wildlife to the euthanasia drug. Leaving a euthanized animal unburied in a field or landfill will put numerous wildlife and even domestic dogs at risk of poisoning. Proper disposal of animal remains is the responsibility of the veterinarian and animal owner. They both may be held accountable for the secondary poisoning of wildlife.
Since 2015, WIL has confirmed pentobarbital poisoning of a bald eagle and several turkey vultures in locations throughout California including Shasta, Marin, Ventura, and Fresno counties. In addition to eagles and vultures, other scavengers such as magpies, crows, ravens, gulls, foxes, coyotes, bobcats, and bears also are at risk of exposure when feeding on contaminated animal remains.
Wildlife that ingest pentobarbital-contaminated animal remains are acutely intoxicated and may appear dead. They often have no reflex response and breathing may be barely detectable. Depending upon how much euthanasia drug has been ingested, the affected wildlife may die at the site of the poisoned carcass or walk or fly short distances and are found debilitated or dead in adjacent fields, near roost trees, or other locations. Intoxication also may predispose the affected wildlife to trauma such as vehicle collisions, drowning, or falling from a perch. More information may be found on the USFWS Secondary Pentobarbital Poisoning of Wildlife (PDF) fact sheet.
|