Abstract
Understanding frequency and variation of cave-exiting activity after arousal from torpor of hibernating bats is important for bat ecology and conservation, especially considering white-nose syndrome. In winter from 2011 to 2018, we acoustically monitored, and counted in hibernacula, two species of conservation concern—western small-footed myotis (Myotis ciliolabrum) and Townsend’s big-eared bats (Corynorhinus townsendii)—in 9 caves located in important habitat for these species in western North America. We investigated if cave-exiting activity differed by species, cave, number of hibernating bats, moon phase, and weather variables. Both species exited hibernacula during all winter months, but most activity occurred in March followed by November. Although we counted almost 15 times more Townsend’s big-eared bats during hibernacula surveys, we documented western small-footed myotis exiting caves 3 times more than Townsend’s big-eared bats. Cave-exiting activity increased with increasing number of hibernating bats, but more so for western small-footed myotis. Both species of bats were active during warm weather and low wind speeds. Western small-footed myotis were more active during colder temperatures, higher wind speeds, and greater change in barometric pressure than Townsend’s big-eared bats. Our results provide a long-term dataset of cave-exiting activity after arousal from torpor during hibernation for these species before the arrival of white-nose syndrome.
Similar content being viewed by others
Introduction
Hibernating bats arouse from torpor (i.e., controlled reductions in body temperature and metabolism to conserve energy) and exit caves during winter because of internal cues and environmental changes1,2,3. Some bats arouse in winter and change positions or fly to another hibernaculum4,5,6, often because other bats arouse and fly7. Female bats arouse for shorter duration than males5,8,9, and younger bats can have shorter torpor bouts than older bats2. Some bats arouse from torpor and fly to potentially find food, water10,11,12, or urinate3; while others try to mate2,4. Arousal from torpor during hibernation may be spontaneous or caused by shifts in temperature and humidity from moving weather fronts4,6,13 and changes in barometric pressure13,14,15. Hibernating bats in temperate environments face selective pressures to budget duration, frequency, and timing of torpor arousals to ensure sufficient energy reserves to support survival and subsequent reproduction7,16,17,18,19.
Humans entering caves for recreation or to conduct research and monitoring can cause bats to arouse from torpor during hibernation20,21,22. Disturbances to bats include lights, noise, vandalism, camping, and caving excursions20,23,24. These disturbances can cause bats to arouse from torpor, elevate body temperatures, and use stored energy reserves; thus potentially reducing winter survival20,23,25. That reduction in survival can impede population growth, because of low annual reproductive rates of bats18,24,26.
A recent threat to bats that causes these mammals to arouse from torpor during hibernation is white-nose syndrome27,28,29. White-nose syndrome is caused by the cold-adapted fungus Pseudogymnoascus destructans30,31. This fungus invades the integumentary system of infected bats causing tissue damage, increased metabolic rate, and water loss because of excessive wing damange27,32,33. Hibernating bats with this disease arouse more often, use more energy because of elevated metabolic rates during torpor, exhibit higher rates of evaporative water loss32,34,35, exit caves more often36,37, and potentially have reduced reproductive success38. Survival of hibernating bats with white-nose syndrome may be influenced by increased arousal and energy expenditure, premature depletion of fat reserves, which can lead to emergence from caves too early and starvation34,38. Additionally, infected bats that arouse and fly more can cause conspecifics to arouse, thus negatively influencing fat stores and survival of both7,39,40. Primarily a disease occurring in eastern North America, white-nose syndrome is now documented in the western USA41.
Bat cave-exiting activity after arousal from torpor during hibernation in western North America is poorly understood42, especially in multiple, adjacent caves. We acoustically monitored, and counted bats in, 9 hibernacula that are in an area of important bat habitat43,44,45 in Idaho, USA, during winter from 2011 to 2018. We hypothesised that cave characteristics, number of hibernating bats in each cave, moon phase, and weather variables would influence nightly cave-exiting activity of hibernating Townsend’s big-eared bats (Corynorhinus townsendii) and western small-footed myotis (Myotis ciliolabrum). Specifically, we predicted that cave-exiting activity would increase for Townsend’s big-eared bats, in large caves, and with more hibernating bats in large clusters10,40,46,47. Also, we predicted that bats would be more active during warm, calm nights3,15, and that those patterns would hold across all caves. These results provide insight into winter behavior of these species and baseline data of cave-exiting activity after arousal from torpor during hibernation prior to the arrival of white-nose syndrome.
Methods
Study area
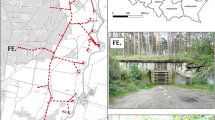
We monitored cave-exiting activity of bats in 9 hibernacula located in an area of roughly 452 km2 on the Snake River Plain in Idaho, USA, on the Idaho National Laboratory Site (43° 36.015 N, 112° 51.441 W). That site was established in the 1940s by the U.S. Atomic Energy Commission as the National Reactor Testing Station, is about 2305 km2, and has been closed to public access since that time48. Caves in our study area were formed from lava blisters produced by pockets of trapped gas or from tubes of molten flows of basaltic lava that were uncovered when the ceiling collapsed49,50. We classified caves as two types: lava blisters or collapsed lava-tubes (Table 1). Lava blisters had small openings (≤ 8 m long x ≤ 6 m wide) in the roof. Conversely, collapsed lava tubes had large openings (≤ 92 m long × 19 m wide) where the roof collapsed forming a crater. Cave ceiling height ranged from about 50 cm to > 10 m. All caves had only one entrance; mean (± SD) cave length was 216 m (± 179.2 m, range 25 to 615 m), and mean elevation at cave openings was 1616 m (± 44 m, range = 1551 to 1701 m). The mean distance from a cave to all other caves was 15 km (SD = 4.6 km). Our study area was a cold desert consisting mainly of sagebrush (Artemisia tridentata)-steppe vegetation44. Weather patterns were hot, dry summers and cold winters48,49. Most precipitation occurred during winter as snow and during spring as rain or snow44. As several of our study caves contain some of the largest hibernating colonies of Townsend’s big-eared bats and western small-footed myotis in western North America43,50, we do not provide cave names to protect those resources25; however, we assigned letter and number combinations to caves that corresponded with cave letter and number combinations in Whiting et al.43. We conducted hibernacula surveys on one day in winter (1 November to 31 March) in 2012, 2013, 2014, 2015, 2017, and 201843,44. Mean date of surveys was February 25. All nine caves were surveyed in a consistent manner each survey. Investigators visually identified and counted bats43,44, and all surveys were performed in accordance to established protocols to minimise disturbance of hibernating bats25,51. Entering caves to count hibernating bats was approved by the Idaho National Laboratory Site Cave Protection and Access Committee (permit number OS-ESD-16-108). That committee oversees, and grants access into, caves for research on the Idaho National Laboratory Site. Townsend’s big-eared bats and western small-footed myotis comprised > 99% of bats observed during hibernacula surveys in our study43.
Passive acoustic sampling
We set acoustic detectors (Anabat SDI and SDII; Titley Scientific, Columbia, MO) outside of caves during winter. All detectors were set within a mean of 3 m (SD = 2.5 m) of the cave opening or the cave lip. Detectors were powered by external batteries and solar panels3,10,52. Each unit was equipped with a protective cover (BatHat) to reduce damage to equipment from rain, snow, and freezing temperatures53; eight directional microphones had reflector plates oriented at 45° angle from the center axis of the microphone3,53,54, and the directional microphone at one cave (C54) did not have a reflector plate, because of unique cave characteristics. Detectors were programmed to record at least from sunset to sunrise16,55,56, and the division ratio was set at eight54. We adjusted the sensitivity to exclude ambient noise57,58,59.
We placed microphones about 3 m above the ground and positioned them so the center axis of the zone of reception was approximately 15° above the horizon15,16,52. We oriented microphones to maximise detection near cave entrances or craters while trying to avoid recording near-ground noise and echoes52,53,58. At collapsed lava-tube caves, we placed detector units near the lip of the crater so that we sampled the area of the crater. At lava blister caves with smaller openings, we set the detector at the cave opening52. When triggered by a bat flying outside of hibernacula, detectors created one, ≤ 15 s. call file, labeled with a date and time stamp.
We filtered call files for bat search-phase calls by species using spectrographic analysis software (AnaLookW10,15,57; Supplementary Table S1). Past studies have successfully recorded and identified Townsend’s big-eared bats and western small-footed myotis with Anabat detectors10,54,58,60. Additionally, one coauthor (Doering who has > 25 years of experience vetting bat calls in the western USA) manually verified species for all files that passed filters. Winter cave-exiting activity after arousal from torpor of bats can be affected when humans enter caves for research and monitoring22,23; in our study, on 17 instances, researchers went into caves on one day to collect samples or conduct hibernacula counts. Therefore, we eliminated data for 24 h after each of those events from our analyses23.
Statistical methods
In our analyses, our response variable was the number of files containing at least one search-phase echolocation sequence of ≥ 2 echolocation pulses for each species15,37,52,53, each night that the detector functioned. That response variable was an index of bat activity and not abundance37,52. Our predictor variables were detector number, year, cave, cave length (m), cave type (lava blister or collapsed lava tube), mean number of hibernating bats counted in each cave, mean cluster size, and mean number of clusters observed during counts in each cave3,37,61. We included mean temperature (°C), mean % relative humidity, mean wind speed (m/s) from ½ hour before sunset to ½ hour after sunrise. We also included maximum minus minimum pressure (hPa) over night, accumulated precipitation (rain and melted snow, mm), and moon phase (fraction of moon illuminated at midnight in Mountain Standard Time, http://aa.usno.navy.mil/data/docs/MoonFraction.php)3,9,16,24,37,62. Weather data were collected from the closest (within 20 km) National Oceanic and Atmospheric Administration weather station to our study caves every 5 min. from 1/2 hour before sunset to 1/2 hour after sunrise each day. Preliminary analyses indicated that cave length was correlated (r > |0.6|) with mean number of Townsend’s big-eared bats and western small-footed myotis counted during surveys. Additionally, three predictor variables—cave length, mean cluster size, and mean number of clusters during surveys—were also confounded with the factor cave, which was required as a random grouping effect in mixed-effect models; therefore, we eliminated those three variables in model building procedures.
We detected bat activity on 19% of nights for Townsend’s big-eared bats and 29% of nights for western small-footed myotis. To account for those data patterns, we used zero-inflated generalised linear mixed models (GLMMs)63,64. We considered the error distributions of GLMMs appropriate for count data. Specifically, we created models that incorporated conventional Poisson errors, as well as negative binomial errors with linear and quadratic parameterization to account for potential overdispersion15,37,65,66. We used a log link for the GLMM mean function for all three error distributions. We also applied three forms of zero inflation in models: no zero-inflation, constant zero-inflation (zero-inflation as a function of the model intercept), and zero-inflation as a function of temperature. The last approach assumed that bat activity occurred based on a minimum temperature threshold. For fixed effects and quantitative predictions in GLMM models, we tested null hypotheses of no effect using Wald tests67. For the random factor cave, we used a likelihood-ratio test for the hypothesis that bat activity did not vary among caves when holding other model terms constant. For all three error distributions and all three forms of zero-inflation, we used backwards stepwise model selection to find optimal approximating (minimum AIC) models68,69. We used R statistical environment for all analyses70 with packages MASS71, asbio67, and glmmTMB72 to create zero-inflated GLMMs. We set alpha at 0.05 for all analyses.
Results
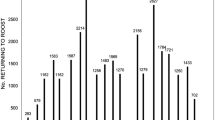
We counted on average almost 15 times more Townsend’s big-eared bats than western small-footed myotis in hibernacula surveys (Table 1). Despite counting more Townsend’s big-eared bats in hibernacula surveys, from 2011 to 2018 at 9 caves, detector units recorded 17,243 files (Townsend’s big-eared bat = 4160 files and western small-footed myotis = 13,083 files; Table 1) during 2204 nights. Mean (± SD) number of files recorded per night across caves for Townsend’s big-eared bats was 2 (± 8.3 files, range = 0 to 220 files) and for western small-footed myotis was = 6 (± 24.0 files, range = 0 to 570 files). We recorded Townsend’s big-eared bats and western small-footed myotis in each month of winter, and western small-footed myotis were recorded on average 3 times more than Townsend’s big-eared bats in each month of winter, except in December (Fig. 1).
The optimal approximating models (i.e., ∆AIC = 0) for activity of Townsend’s big-eared bats and western small-footed myotis in winter were similar and both contained the same 11 predictor variables (Supplementary Tables S2, S3). For Townsend’s big-eared bats, temperature was the strongest predictor, followed by wind, barometric pressure, and number of hibernating bats in caves (Table 2). For western small-footed myotis, temperature was the strongest predictor, followed by wind, year, number of hibernating bats in caves, and barometric pressure (Table 3). Drop in deviance tests for the random factor cave indicated that activity of Townsend’s big-eared bats (σ2REML = 0.52, X2 = 67.1, p < 0.001) and western small-footed myotis (σ2REML = 0.52, X2 = 47.7, p < 0.001) varied widely across caves. Both species were more active during warm weather, low wind speeds, and greater change in barometric pressure (Fig. 2); western small-footed myotis were more active at colder temperatures, higher wind speeds, greater change in barometric pressure, and when more bats were counted during hibernation than Townsend’s big-eared bats (Fig. 2). At 0 °C, predicted bat activity was 1.9 files/night for Townsend’s big-eared bats and 5.9 files/night for western small-footed myotis (Fig. 2a). At 10 °C, predicted activity increased to 8.5 files/night for Townsend’s big-eared bats and 31.7 files/night for western small-footed myotis (Fig. 2a).
Fitted models while holding predictor variables constant for bat activity (files/night) in 9 hibernacula for Townsend’s big-eared bats (Corynorhinus townsendii) and western small-footed myotis (Myotis ciliolabrum) by (a) temperature, (b) wind speed, (c) barometric pressure, and (d) number of hibernating bats in caves in southeastern Idaho, USA, from 2011 to 2018.
Discussion
Biologists need to understand species-specific differences in bat winter ecology that can influence mortality risk for hibernating bats, especially in western North America3,42. We predicted that cave-exiting behavior after arousal from torpor would increase for the larger sized Townsend’s big-eared bats10. Although we counted almost 15 times more of that species than western small-footed myotis during hibernacula surveys, because small-footed myotis are more difficult to observe when hibernating73,74, we documented western small-footed myotis exiting caves after arousal from torpor during hibernation on average 3 times more than Townsend’s big-eared bats. Different arousal and flying patterns during winter have been documented for other species hibernating together3,75,76, and generally it takes more energy for larger bats to arouse and fly than smaller bats20,77. Also, difficulty exists when comparing winter activity of bat species using acoustic recordings, because of species-specific differences in intensity of echolocation calls and atmospheric attenuation57,61,78, especially for Townsend’s big-eared bats as their calls are lower intensity compared with calls of western small-footed myotis52,60. Such differences need to be considered when comparing acoustic activity between species. Nonetheless, understanding frequency and variation of bat winter activity levels across species is important for bat ecology and conservation10,76,79, especially in light of white-nose syndrome9,80. General conditions of humidity and temperature exist for growth of Pseudogymnoascus destructans in the western USA36,50, and Townsend’s big-eared bats can carry this fungus81. Our results provide long-term baseline data of cave-exiting activity prior to the arrival white-nose syndrome in Idaho, which can be used to compare with changes in bat activity after this fungus arrives as has been done in the eastern USA80,82,83.
One of the main differences in winter cave-exiting activity between the two species we studied could have been due to differences in body size and evaporative water loss. Mass of adult Townsend’s big-eared bats ranges from 5 to 13 g, and females are heavier than males in autumn and winter4,6. For western small-footed myotis, mass of adult animals is about 4.5 g84. Smaller bats may arouse more from torpor during hibernation to drink water. Indeed, a laboratory study indicated that bats must drink every 9–12 days during hibernation77, and studies conducted in the field have provided evidence of bat arousing from torpor in winter to drink water10. Also, bats that arouse more during hibernation have higher rates of total evaporative water loss12, and evaporative water loss may also be driven by humidity levels in caves12.
Little is known about bat cave-exiting activity after arousal from torpor among multiple hibernacula with differing numbers of bats. Our prediction that cave-exiting behavior would increase in large caves with more hibernating bats was upheld. Indeed, we documented a positive trend in bat activity by both species with increasing number of hibernating conspecifics in caves, but more so for western small-footed myotis. Large numbers of bats and groups of conspecifics can cause other bats to arouse and fly in winter40,75, which may have occurred in our study area. We hypothesise that when white-nose syndrome arrives in Idaho, infected bats in caves with more hibernating individuals will cause conspecifics to arouse more, thus negatively impacting survival of both7,39,40. Our data also indicated that cave-exiting behavior varied widely across the 9 caves for western small-footed myotis and Townsend’s big-eared bats. Observed differences in cave-exiting behavior highlights the importance of quantifying bat activity at caves with differing number of hibernating bats to understand the influence of habitat and environmental variables, as well as disease, on local bat populations52.
We documented highest levels of bat activity during November and March, and lowest levels during the coldest winter months of December, January, and February, which has been documented in other studies in temperate, northern environments10,16,52. Also, less variation was evident in activity of Townsend’s big-eared bats both within and among months. Bats often fly and forage at the beginning and end of hibernation season on warm, calm nights77. During the coldest months of winter, however, bats go farther into caves6,24. Timing arousal events to coincide with high ambient temperatures reduces the total energy expense of reaching euthermia3. Indeed, relying on increased ambient temperature to elevate body temperature (passive rewarming) can save 20% of the energetic cost of arousal85. Species that hibernate assess environmental conditions at or near the entrances of hibernacula to more accurately time emergence. Also, changes in barometric pressure could signal favorable conditions for bat emergence, especially for individuals that roost deep in caves13,86. The more frequently that this assessment is done, the more accurately that emergence can be timed47.
We predicted that bats would be more active during warm, calm nights. In our study, temperature, wind, and change in barometric pressure were strong predictors of bat activity for both species; however, western small-footed myotis were more active at colder temperatures, higher wind speeds, and higher change in barometric pressure than Townsend’s big-eared bats. Our result differed from another study that documented the larger sized big brown bat (Eptesicus fuscus) as more active at higher temperatures than Myotis spp.15. Temperature and wind speed were predictors of bat activity in other studies13,15,62, and bats responded to weather patterns62 and changes in barometric pressure13,15. Bat calls have been recorded at temperatures below 0 °C15, but most activity occurred on nights when the temperature at sunset exceeded 0 °C10,62, and the probability of activity increases as temperature increases62, similar to what we documented. Warmer ambient temperatures can also increase frequency of arousals within hibernacula; we were not able to relate the frequency of arousals of bats within hibernacula to activity outside of those features, because we did not acoustically monitor bats inside hibernacula. Future studies need to test how cave-exiting activity by bats relates to frequency of arousals and bats flying in hibernacula.
Much interest exists in developing long-term acoustic monitoring of bats56,82,87, and deploying several stationary detectors is valuable for understanding bat activity at a landscape scale61. With the arrival of white-nose syndrome in western North America41, it is important to understand cave-exiting behavior of bats after arousal from torpor9,75. Furthermore, comparisons among species need to be conducted at large geographic scales to determine differences in winter activity strategies88. We acoustically monitored, and counted bats in, 9 hibernacula that were in an area of important habitat during winter. We recorded western small-footed myotis exiting caves 3 times more than Townsend’s big-eared bats, and cave-exiting behavior increased similarly with increasing number of hibernating bats for these species. Temperature, wind speed, and change in barometric pressure were strong predictors of bat activity for both species. Our results provide insight into cave-exiting activity after arousal from torpor of these species and provide a long-term baseline dataset of that activity prior to the arrival of white-nose syndrome. Such data can help biologists when quantifying the potential impact of white-nose syndrome on these species.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author.
References
Hope, P. R. & Jones, G. Warming up for dinner: Torpor and arousal in hibernating Natterer’s bats (Myotis nattereri) studied by radio telemetry. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 182, 569–578. https://doi.org/10.1007/s00360-011-0631-x (2012).
Czenze, Z. J., Jonasson, K. A. & Willis, C. K. R. Thrifty females, frisky males: Winter energetics of hibernating bats from a cold climate. Physiol. Biochem. Zool. 90, 502–511. https://doi.org/10.1086/692623 (2017).
Reynolds, D. S., Shoemaker, K., von Oettingen, S. & Najjar, S. High rates of winter activity and arousals in two New England bat species: Implications for a reduced white-nose syndrome impact?. Northeast. Nat. 24, B188–B208 (2017).
Kunz, T. H. & Martin, R. A. Plecotus townsendii. Mamm. Species 175, 1–6 (1982).
Twente, J. W. Aspects of a population study of cavern-dwelling bats. J. Mamm. 36, 379–390 (1955).
Humphrey, S. R. & Kunz, T. H. Ecology of a Pleistocene relict, the western big-eared bat (Plecotus townsendii), in the southern Great Plains. J. Mamm. 57, 470–494. https://doi.org/10.2307/1379297 (1976).
Czenze, Z. J., Park, A. D. & Willis, C. K. R. Staying cold through dinner: Cold-climate bats rewarm with conspecifics but not sunset during hibernation. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 183, 859–866. https://doi.org/10.1007/s00360-013-0753-4 (2013).
Pearson, O. P., Koford, M. R. & Pearson, A. K. Reproduction of the lump-nosed bat (Corynorhinus rafinesquei) in California. J. Mamm. 33, 273–320 (1952).
Johnson, J. S., Lacki, M. J., Thomas, S. C. & Grider, J. F. Frequent arousals from winter torpor in Rafinesque’s big-eared bat (Corynorhinus rafinesquii). PLoS ONE 7, e49754. https://doi.org/10.1371/journal.pone.0049754 (2012).
Lausen, C. L. & Barclay, R. M. R. Winter bat activity in the Canadian prairies. Can. J. Zool.-Rev. Can. Zool. 84, 1079–1086. https://doi.org/10.1139/z06-093 (2006).
Thomas, D. W. & Cloutier, D. Evaporative water-loss by hibernating little brown bats, Myotis lucifugus. Physiol. Zool. 65, 443–456 (1992).
Ben-Hamo, M., Munoz-Garcia, A., Williams, J. B., Korine, C. & Pinshow, B. Waking to drink: Rates of evaporative water loss determine arousal frequency in hibernating bats. J. Exp. Biol. 216, 573–577. https://doi.org/10.1242/jeb.078790 (2013).
Czenze, Z. J. & Willis, C. K. R. Warming up and shipping out: Arousal and emergence timing in hibernating little brown bats (Myotis lucifugus). J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 185, 575–586. https://doi.org/10.1007/s00360-015-0900-1 (2015).
Choate, J. R. & Anderson, J. M. Bats of jewel cave national monument, South Dakota. Prairie Nat. 29, 39–47 (1997).
Klüg-Baerwald, B. J., Gower, L. E., Lausen, C. L. & Brigham, R. M. Environmental correlates and energetics of winter flight by bats in southern Alberta, Canada. Can. J. Zool. 94, 829–836. https://doi.org/10.1139/cjz-2016-0055 (2016).
Johnson, J. S. et al. Migratory and winter activity of bats in Yellowstone National Park. J. Mamm. 98, 211–221. https://doi.org/10.1093/jmammal/gyw175 (2017).
Norquay, K. & Willis, C. Hibernation phenology of Myotis lucifugus. J. Zool. 294, 85–92 (2014).
Barclay, R. M. et al. Variation in the reproductive rate of bats. Can. J. Zool. 82, 688–693 (2004).
Jonasson, K. A. & Willis, C. K. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE 6, e21061. https://doi.org/10.1371/journal.pone.0021061 (2011).
Speakman, J. R., Webb, P. I. & Racey, P. A. Effects of disturbance on the energy expenditure of hibernating bats. J. Appl. Ecol. 28, 1087–1104. https://doi.org/10.2307/2404227 (1991).
Reeder, D. M., Field, K. A. & Slater, M. H. Balancing the costs of wildlife research with the benefits of understanding a panzootic disease, white-nose syndrome. ILAR J. 56, 275–282. https://doi.org/10.1093/ilar/ilv035 (2015).
Boyles, J. G. Benefits of knowing the costs of disturbance to hibernating bats. Wildl. Soc. Bull. 41, 388–392. https://doi.org/10.1002/wsb.755 (2017).
Thomas, D. W. Hibernating bats are sensitive to nontactile human disturbance. J. Mamm. 76, 940–946. https://doi.org/10.2307/1382764 (1995).
Furey, N. M. & Racey, P. A. Bats in the Anthropocene: Conservation of Bats in a Changing World 463–500 (Springer, 2016).
Sheffield, S. R., Shaw, J. H., Heidt, G. A. & McClenaghan, L. R. Guidelines for the protection of bat roosts. J. Mamm. 73, 707–710 (1992).
Jones, G., Jacobs, D. S., Kunz, T. H., Willig, M. R. & Racey, P. A. Carpe noctem: The importance of bats as bioindicators. Endang. Species Res. 8, 93–115 (2009).
Blehert, D. S. et al. Bat white-nose syndrome: An emerging fungal pathogen?. Science 323, 227. https://doi.org/10.1126/science.1163874 (2009).
Foley, J., Clifford, D., Castle, K., Cryan, P. & Ostfeld, R. S. Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conserv. Biol. 25, 223–231. https://doi.org/10.1111/j.1523-1739.2010.01638.x (2011).
Ingersoll, T. E., Sewall, B. J. & Amelon, S. K. Effects of white-nose syndrome on regional population patterns of 3 hibernating bat species. Conserv. Biol. 30, 1048–1059. https://doi.org/10.1111/cobi.12690 (2016).
Minnis, A. M. & Lindner, D. L. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 117, 638–649. https://doi.org/10.1016/j.funbio.2013.07.001 (2013).
Lorch, J. M. et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376 (2011).
Verant, M. L. et al. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 14, 10 (2014).
Warnecke, L. et al. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl. Acad. Sci. U.S.A. 109, 6999–7003. https://doi.org/10.1073/pnas.1200374109 (2012).
Lilley, T. M. et al. White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Front. Zool. https://doi.org/10.1186/s12983-016-0143-3 (2016).
McGuire, L. P., Mayberry, H. W. & Willis, C. K. R. White-nose syndrome increases torpid metabolic rate and evaporative water loss in hibernating bats. Am. J. Physiol.-Regulat. Integr. Compar. Physiol. 313, R680–R686. https://doi.org/10.1152/ajpregu.00058.2017 (2017).
Knudsen, G. R., Dixon, R. D. & Amelon, S. K. Potential spread of white-nose syndrome of bats to the Northwest: Epidemiological considerations. Northwest Sci. 87, 292–306. https://doi.org/10.3955/046.087.0401 (2013).
Bernard, R. F. & McCracken, G. F. Winter behavior of bats and the progression of white-nose syndrome in the southeastern United States. Ecol. Evol. 7, 1487–1496. https://doi.org/10.1002/ece3.2772 (2017).
Cheng, T. L. et al. Higher fat stores contribute to persistence of little brown bat populations with white-nose syndrome. J. Anim. Ecol. 88, 591–600 (2019).
Turner, J. M. et al. Conspecific disturbance contributes to altered hibernation patterns in bats with white-nose syndrome. Physiol. Behav. 140, 71–78 (2015).
Blazek, J. et al. Numerous cold arousals and rare arousal cascades as a hibernation strategy in European Myotis bats. J. Therm. Biol 82, 150–156. https://doi.org/10.1016/j.jtherbio.2019.04.002 (2019).
Lorch, J. M. et al. First detection of bat white-nose syndrome in Western North America. mSphere 1(4), e00148. https://doi.org/10.1128/mSphere.00148-16 (2016).
Weller, T. J. et al. A review of bat hibernacula across the western United States: Implications for white-nose syndrome surveillance and management. PLoS ONE https://doi.org/10.1371/journal.pone.0205647 (2018).
Whiting, J. C. et al. Bat hibernacula in caves of southern Idaho: Implications for monitoring and management. West. N. Am. Nat. 78, 165–173 (2018).
Whiting, J. C. et al. Long-term bat abundance in sagebrush steppe. Sci. Rep. 8, 12288 (2018).
Call, R. S. et al. Maternity roosts of Townsend’s big-eared bats in lava tube caves of southern Idaho. Northwest Sci. 92, 158–165 (2018).
Clark, B. S., Clark, B. K. & Leslie, D. M. Seasonal variation in activity patterns of the endangered Ozark big-eared bat (Corynorhinus townsendii ingens). J. Mamm. 83, 590–598. https://doi.org/10.1644/1545-1542(2002)083%3c0590:sviapo%3e2.0.co;2 (2002).
French, A. R. The patterns of mammalian hibernation. Am. Sci. 76, 568–575 (1988).
Reynolds, T. D., Connelly, J. W., Halford, D. K. & Arthur, W. J. Vertebrate fauna of the Idaho National Environmental Research Park. Gt. Basin Nat. 46, 513–527 (1986).
Genter, D. L. Wintering bats of the upper Snake River Plain: Occurrence in lava-tube caves. Gt. Basin Nat. 46, 241–244 (1986).
Gillies, K. E., Murphy, P. J. & Matocq, M. D. Hibernacula characteristics of Townsend’s big-eared bats in southeastern Idaho. Nat. Areas J. 34, 24–30 (2014).
Sikes, R. S. et al. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mamm. 97(663–688), 2016. https://doi.org/10.1093/jmammal/gyw078 (2016).
Schwab, N. A. & Mabee, T. J. Winter acoustic activity of bats in Montana. Northwest. Nat. 95, 13–27 (2014).
Britzke, E. R., Slack, B. A., Armstrong, M. P. & Loeb, S. C. Effects of orientation and weatherproofing on the detection of bat echolocation calls. J. Fish Wildl. Manage. 1, 136–141. https://doi.org/10.3996/072010-jfwm-025 (2010).
Skalak, S. L., Sherwin, R. E. & Brigham, R. M. Sampling period, size and duration influence measures of bat species richness from acoustic surveys. Methods Ecol. Evol. 3, 490–502. https://doi.org/10.1111/j.2041-210X.2011.00177.x (2012).
Miller, B. W. A method for determining relative activity of free flying bats using a new activity index for acoustic monitoring. Acta Chiropt. 3, 93–105 (2001).
Nocera, T., Ford, W. M., Silvis, A. & Dobony, C. A. Patterns of acoustical activity of bats prior to and 10 years after WNS on Fort drum army installation, New York. Glob. Ecol. Conserv. https://doi.org/10.1016/j.gecco.2019.e00633 (2019).
Britzke, E. R., Gillam, E. H. & Murray, K. L. Current state of understanding of ultrasonic detectors for the study of bat ecology. Acta Theriol. 58, 109–117. https://doi.org/10.1007/s13364-013-0131-3 (2013).
O’Farrell, M. J., Miller, B. W. & Gannon, W. L. Qualitative identification of free-flying bats using the Anabat detector. J. Mamm. 80, 11–23. https://doi.org/10.2307/1383203 (1999).
Whiting, J. C., Doering, B. & Pennock, D. Acoustic surveys for local, free-flying bats in zoos: An engaging approach for bat education and conservation. J. Bat Res. Conserv. 12, 94–99. https://doi.org/10.14709/BarbJ.12.1.2019.12 (2019).
O’Farrell, M. J. & Gannon, W. L. A comparison of acoustic versus capture techniques for the inventory of bats. J. Mamm. 80, 24–30. https://doi.org/10.2307/1383204 (1999).
Stahlschmidt, P. & Bruhl, C. A. Bats as bioindicators—The need of a standardized method for acoustic bat activity surveys. Methods Ecol. Evol. 3, 503–508. https://doi.org/10.1111/j.2041-210X.2012.00188.x (2012).
Avery, M. I. Winter activity of pipistrelle bats. J. Anim. Ecol. 54, 721–738. https://doi.org/10.2307/4374 (1985).
McCulloch, C. E. & Neuhaus, J. M. Generalized linear mixed models. In Encyclopedia of Biostatistics (eds Armitage, P. & Colton, T.) (Wiley, 2005).
Nelder, J. A. & Wedderburn, R. W. Generalized linear models. J. R. Stat. Soc. Ser. A (Gen.) 135, 370–384 (1972).
Hardin, J. W. & Hilbe, J. M. Generalized Linear Models and Extensions (Stata Press, 2007).
Consul, P. & Famoye, F. Generalized Poisson regression model. Commun. Stat. Theory Methods 21, 89–109 (1992).
Aho, K. A. Foundational and Applied Statistics for Biologists using R (CRC Press, 2013).
Akaike, H. Selected Papers of Hirotugu Akaike 199–213 (Springer, 1998).
Burnham, K. P. & Anderson, D. A. Model Selection and Multimodel Inference: A practical Information-Theoretic Approach 2nd edn. (Springer, 2002).
RCoreTeam. R: A Language and Environment for Statistical Computing (2020).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S-PLUS (Springer, 2013).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Perkins, J. M., Barss, J. M. & Peterson, J. Winter records of bats in Oregon and Washington. Northwest. Nat. 71, 59–62. https://doi.org/10.2307/3536594 (1990).
Nagorsen, D. W. et al. Winter bat records for British Columbia. Northwest Nat. 74, 61–66 (1993).
Hayman, D. T., Cryan, P. M., Fricker, P. D. & Dannemiller, N. G. Long-term video surveillance and automated analyses reveal arousal patterns in groups of hibernating bats. Methods Ecol. Evol. 8, 1813–1821 (2017).
Boyles, J. G., Dunbar, M. B. & Whitaker, J. O. Activity following arousal in winter in North American vespertilionid bats. Mamm. Rev. 36, 267–280. https://doi.org/10.1111/j.1365-2907.2006.00095.x (2006).
Speakman, J. R. & Racey, P. A. Hibernal ecology of the pipistrelle bat: Energy expenditure, water requirements and mass-loss, implications for survial and the function of winter emergence flights. J. Anim. Ecol. 58, 797–813. https://doi.org/10.2307/5125 (1989).
Lawrence, B. D. & Simmons, J. A. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J. Acoust. Soc. Am. 71, 585–590 (1982).
Dunbar, M. B. & Tomasi, T. E. Arousal patterns, metabolic rate, and an energy budget of eastern red bats (Lasiurus borealis) in winter. J. Mamm. 87, 1096–1102. https://doi.org/10.1644/05-mamm-a-254r3.1 (2006).
Ford, W. M., Britzke, E. R., Dobony, C. A., Rodrigue, J. L. & Johnson, J. B. Patterns of acoustical activity of bats prior to and following white-nose syndrome occurrence. J. Fish Wildl. Manage. 2, 125–134. https://doi.org/10.3996/042011-jfwm-027 (2011).
Bernard, R. F., Foster, J. T., Willcox, E. V., Parise, K. L. & McCracken, G. F. Molecular detection of the causative agent of white-nose syndrome on Rafinesque’s big-eared bats (Corynorhinus rafinesquii) and two species of migratory bats in the southeastern USA. J. Wildl. Dis. 51, 519–522. https://doi.org/10.7589/2014-08-202 (2015).
Dzal, Y., McGuire, L. P., Veselka, N. & Fenton, M. B. Going, going, gone: the impact of white-nose syndrome on the summer activity of the little brown bat (Myotis lucifugus). Biol. Lett. 7, 392–394 (2010).
Brooks, R. T. Declines in summer bat activity in central New England 4 years following the initial detection of white-nose syndrome. Biodivers. Conserv. 20, 2537–2541. https://doi.org/10.1007/s10531-011-9996-0 (2011).
Holloway, G. L. & Barclay, R. M. R. Myotis ciliolabrum. Mamm. Species 670, 1–5. https://doi.org/10.1644/1545-1410(2001)670%3c0001:mc%3e2.0.co;2 (2001).
Halsall, A. L., Boyles, J. G. & Whitaker, J. O. Jr. Body temperature patterns of big brown bats during winter in a building hibernaculum. J. Mamm. 93, 497–503 (2012).
Paige, K. N. Bats and barometric pressure: conserving limited energy and tracking insects from the roost. Funct. Ecol. 9, 463–467 (1995).
Frick, W. F. Acoustic monitoring of bats, considerations of options for long-term monitoring. Therya 4, 69–78 (2013).
Whitaker, J. O. & Rissler, L. J. Winter activity of bats at a mine entrance in Vermillion County, Indiana. Am. Midl. Nat. 127, 52–59. https://doi.org/10.2307/2426321 (1992).
Acknowledgements
We thank employees of Wastren Advantage. We also thank the US Department of Energy, Idaho Operations Office at the INL Site for funding (contract number DE-NE0008477).
Author information
Authors and Affiliations
Contributions
J.C.W. and B.D. designed the study. J.C.W. and B.D. performed field work. J.C.W., B.D. and K.A. analysed data. J.C.W. wrote an initial draft of the manuscript and all authors finalised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whiting, J.C., Doering, B., Aho, K. et al. Long-term patterns of cave-exiting activity of hibernating bats in western North America. Sci Rep 11, 8175 (2021). https://doi.org/10.1038/s41598-021-87605-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87605-0
This article is cited by
-
Artificial light at night (ALAN) pollution alters bat lunar chronobiology: insights from broad-scale long-term acoustic monitoring
Ecological Processes (2024)
-
How weather triggers the emergence of bats from their subterranean hibernacula
Scientific Reports (2023)
-
How many nights should acoustic detectors be set to estimate cave-exiting behavior of hibernating bats?
Mammal Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.